Obesity is not merely an excess of weight; it is a complex disease associated with a chronic, low-grade inflammatory state—silent yet persistent—that contributes to the development of type 2 diabetes, hypertension, fatty liver, and cardiovascular disease. This state arises from the expansion of adipose tissue, which acts as a metabolically active organ capable of releasing adipokines and pro-inflammatory cytokines, sustaining systemic inflammation that increases cardiometabolic risk even in the absence of symptoms.
Although traditional markers such as C-reactive protein (CRP) or high-sensitivity CRP (hsCRP) can reflect part of this process, their high interindividual variability and sensitivity to acute fluctuations limit their clinical usefulness.
In this context, a new study published by Mercedes Bernaldo de Quirós Fernández, Elías Rodríguez Cuellar, Blanca Otero Torrón, Nuria Amigó, Eduardo Ferrero Herrero, Cristina Martín‐Arriscado Arroba, and Ana Isabel Pérez Zapata in Obesity Surgery demonstrates that nuclear magnetic resonance (NMR) spectroscopy and glycoprotein-derived biomarkers — GlycA, GlycB, and GlycF — allow for precise evaluation of this inflammatory state and monitoring of its reduction after metabolic surgery. This approach offers a more stable, reproducible, and sensitive characterization compared to conventional markers.
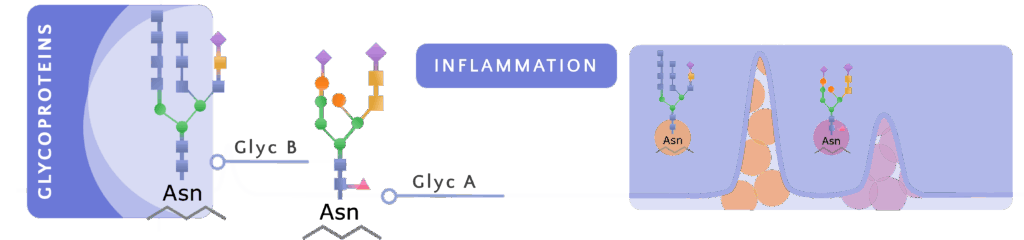
Acute-phase proteins have characteristic glycan structures — N-acetylglucosamine and N-acetylgalactosamine (GlycA) and N-acetylneuraminic acid (GlycB) — that can be directly quantified in plasma using NMR spectroscopy. Measuring GlycA and GlycB reflects an integrated set of pro-inflammatory proteins, rather than a single isolated marker, providing a more robust and reproducible assessment of systemic inflammation.
Unlike CRP and hsCRP, whose levels show greater interindividual variability and lower reliability in predicting cardiovascular risk, NMR-derived glycoprotein signals offer analytical stability and lower susceptibility to acute fluctuations, capturing low-grade chronic inflammation more faithfully.
The H/W (height/width) indices of GlycA and GlycB allow deeper interpretation of the inflammatory profile: the peak height reflects the concentration of acute-phase proteins, while the width is related to their aggregation state and the stability of glycosidic bonds. Thus, higher and narrower peaks are associated with a more pronounced pro-inflammatory state.
Meanwhile, GlycF represents N-acetyl groups not bound to proteins; although its role is less defined, it complements the overall characterization of the patient’s inflammatory state.
The study analyzed 52 patients with obesity who underwent metabolic surgery, including Roux-en-Y gastric bypass (RYGB), omega-loop gastric bypass (BRYGB), and sleeve gastrectomy (SG). The average age was 46 years, with a preoperative BMI of 43.6 kg/m², and the follow-up lasted 18 months.
The biomarkers GlycA, GlycB, GlycF, and H/W indices were quantified using ¹H NMR spectroscopy on a Bruker Avance III 600 MHz spectrometer, both before the intervention and at the end of follow-up. Since each patient served as their own control, the impact of surgery on systemic inflammation could be directly measured.
The results showed a marked and sustained reduction in low-grade inflammation after metabolic surgery, with highly significant decreases in GlycA, GlycB, and GlycF — all displaying exceptionally large effect sizes (Cohen’s d > 1.8)— regardless of the surgical technique used.
This profound decrease indicates that surgery not only contributes to weight loss but also intensely modulates the systemic inflammatory state, a key component of cardiometabolic risk. Advanced characterization via NMR enables precise monitoring of this evolution, detection of residual risk profiles, and guidance of personalized interventions during clinical follow-up.
Overall, the findings reinforce the value of NMR-derived glycoproteins as tools to monitor metabolic benefits beyond weight reduction, providing a more comprehensive and sensitive view of the true impact of metabolic surgery on inflammatory and cardiovascular health.
Read the full article here.